Developing Homogeneous Catalysts for Efficient Water Splitting:
A Collaboration with the MAXNET Energy Consortium
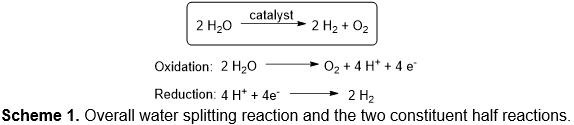
While there are many challenges to large-scale utilization of solar energy, non-biological water splitting that couples light harvesting, the oxidation of water to dioxygen, and the reduction of protons to dihydrogen is a promising strategy. Dihydrogen could be used directly in fuel cells or converted into hydrogen-rich carbon-based liquid fuels (e.g., alcohols or hydrocarbons via reduction of carbon dioxide or oxygen-rich biomass). One strategy to accomplish solar driven water splitting is to convert solar energy to electricity to drive electrocatalytic processes. The water splitting reaction is divided into two redox half-reactions (Scheme 1). Although both half-reactions present challenges, it is recognized that the oxygen evolving reaction (OER) is likely the most difficult, and a central fundamental chemical challenge for the water oxidation reaction is the formation of the O−O bond.
The design of devices aimed at energy harvesting, based on solar energy driven water splitting, requires efficient water oxidation catalysts (WOCs). Currently, electrocatalytic water oxidation is typically accompanied with significant overpotential (i.e., applied driving force beyond the thermodynamic water oxidation potential; EoH2O/O2) to achieve reasonable current densities. Our group's focus on molecular WOCs seeks to further the understanding of the OER mechanism and to elucidate the interplay between catalyst structure, catalytic activity and OER overpotential. Moreover, molecular systems provide a greater ease of study and can further the understanding of abiotic heterogeneous catalysts, which are much more difficult to characterize and study at a molecular level. With increasingly sophisticated methods for preparing and characterizing heterogeneous catalysts, it is becoming more feasible to consider the translation of knowledge gained from study of molecular catalysts to improved heterogeneous catalysts. Thus, we are working within the MAXNET Energy effort (Figure 1), which is a consortium of eight Max Planck Institutes, Cardiff University, and the University of Virginia to develop and understand efficient catalysts for oxidation processes (click here for more information).

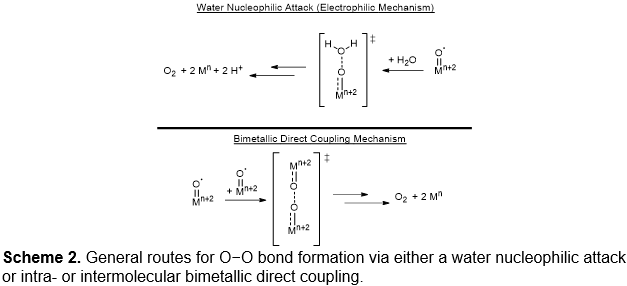
Our group is currently pursuing strategies for durable solution-phase catalysis that facilitate intrametallic O−O bond forming using either 1) multinuclear complexes, based on Cu and Co, as opposed to generating highly electrophilic metal-oxo moieties that undergo water nucleophilic attack (Scheme 2) or 2) redox non-innocent ligands. These strategies permit distribution of oxidizing equivalents across multiple metal/ligand sites; thereby circumventing highly electrophilic intermediates that can undergo oxidative degradation through H-atom abstraction.
Representative Publications:
1. "Immobilization of "Capping Arene" Cobalt(II) Complexes on Ordered Mesoporous Carbon for Electrocatalytic Water Oxidation" Liu, C., Geer, A. M., Webber, C., Musgrave III, C. B., Gu, S., Johnson, G., Dickie, D. A., Chabbra, S., Schnegg, A., Zhou, H., Sun, C.-J., Hwang, S., Goddard III, W. A.*, Zhang, S.*, Gunnoe, T. B.* ACS Catal. 2021, 11, 15068-15082. DOI: 10.1021/acscatal.1c04617
2. "Non-covalent Immobilization of Pentamethylcyclopentadienyl Iridium Complexes on Ordered Mesoporous Carbon for Electrocatalytic Water Oxidation" Geer, A. M., Liu, C., Musgrave, C., Webber, C., Johnson, G., Zhou, H., Sun, C-J., Dickie, D. A., Goddard III, W. A.*, Zhang, S.*, Gunnoe, T. B.* Small Science 2021, 1, 2100037, 1-12. DOI: 10.1002/smsc.202100037
3. "Electrocatalytic Water Oxidation by a Trinuclear Copper(II) Complex" Geer, A. M., Musgrave C., Webber, C., Nielsen, R. J., McKeown, B. A., Liu, C., Schleker, P. P. M., Jakes, P., Jia, X., Dickie, D. A., Granwehr, J., Zhang, S., Machan, C. W., Goddard III, W. A., Gunnoe, T. B. ACS Catalysis 2021, 11, 7223-7240. DOI: 10.1021/acscatal.1c01395