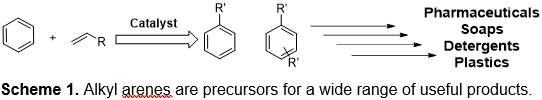
Billions of pounds of alkyl and alkenyl arenes are produced each year. The alkyl and alkenyl arenes are precursors for plastics, elastomers, detergents and pharmaceuticals (Scheme 1). Linear alkyl benzenes (LABs), which are widely-used starting materials for surfactants, are long-chain alkyl benzenes that typically consist of 2- and 3-phenyl alkanes. Truly linear (i.e., straight-chain alkyl) alkylbenzenes, 1-phenyl alkanes, are potential new precursors for soaps and detergent that could offer improved biodegradability. In addition, 1-phenyl alkanes have been reported to have enhanced detersive power relative to 2- and 3-substituted LABs.
Currently, the dominant method to produce alkyl benzenes involves acid promoted aromatic alkylation using olefins. Catalysts are based on traditional Friedel-Crafts processes (e.g., AlCl3 and either HCl or HF) or acidic zeolites. However, these processes have drawbacks that result from the mechanism (i.e., electrophilic aromatic substitution). These include substituent directed regioselectivity (versus catalytic directed selectivity) for the production of di-substituted arenes, sluggish reactivity with electron-deficient arenes, and the inability to produce 1-aryl alkanes. Transition metal-mediated olefin hydroarylation or oxidative olefin hydroarylation that proceeds through arene C–H activation and olefin insertion into metal-aryl bonds offers potential advantages2,3,11.
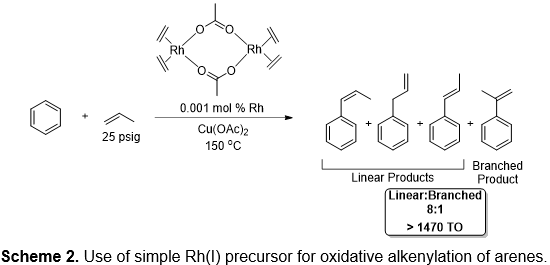
Our group has developed a series of catalysts for arene alkylation or alkenylation, including catalysts based on Ru1-6, Pt7,8 and Rh9-11. Recently, we have reported that Rh(I) complexes can catalyze the single step conversion of benzene and olefins to alkenyl arenes with high turnover number.9-11 Also, the Rh(I) based catalysts can also be used to produce 1-phenyl substituted alkene products via oxidative arene alkenylation using arene and long-chain α-olefins (Scheme 2).3 We have labeled the hydrogenation products "super linear alkyl benzenes" (SLABs) to differentiate them from the branched LABs. We are currently extending the work to understand the mechanism of these reactions, to develop new molecular catalysts, and to develop heterogeneous variants.
References
(1) N.A. Foley, M. Lail, J.P. Lee, T.B. Gunnoe, T.R. Cundari, J.L. Petersen, J. Am. Chem. Soc. 2007, 129, 6765-6781.
(2) M. Lail, B.N. Arrowood, T.B. Gunnoe, J. Am. Chem. Soc. 2003 125, 7506-7507.
(3) M. Lail, C.M. Bell, D. Conner, T.R. Cundari, T.B. Gunnoe, J.L. Petersen, Organometallics 2004, 23, 5007-5020.
(4) N.A. Foley, M. Lail, T.B. Gunnoe, T.R. Cundari, P.D. Boyle, J.L. Petersen, Organometallics 2007, 26, 5507-5516.
(5) E.E. Joslin, C.L. McMullin, T.B. Gunnoe, T.R. Cundari, M. Sabat, W.H. Myers, Organometallics 2012, 31, 6851-6860.
(6) N.A. Foley, Z.F. Ke, T.B. Gunnoe, T.R. Cundari, J.L. Petersen, Organometallics 2008, 27, 3007-3017.
(7) B.A. McKeown, H.E. Gonzalez, M.R. Friedfeld, T.B. Gunnoe, T.R. Cundari, M. Sabat, J. Am. Chem. Soc. 2011, 133, 19131-19152
(8) B.A. McKeown, N.A. Foley, J.P. Lee, T.B. Gunnoe, Organometallics 2008, 27, 4031-4033.
(9) Vaughan, B. A. Vaughan, M. S. Webster-Gardiner, T. R. Cundari, T. B. Gunnoe, Science 2015, 348, 421-424.
(10) B. A. Vaughan, S. K. Khani, J. B. Gary, J. D. Kammert, M. S. Webster-Gardiner, B. A. McKeown, R. J. Davis, T. R. Cundari, T. B. Gunnoe, J. Am. Chem. Soc. 2017, 139, 1485-1498.
(11) M. S. Webster-Gardiner, J. Chen, B. A. Vaughan, B. A. McKeown, W. Schinski, T. B. Gunnoe, J. Am. Chem. Soc. 2017, 139, 5474-5480.