Carbon dioxide is a possible C1 synthon with low cost, high accessibility and the opportunity for recyclability. Despite the possible benefits for the utilization of carbon dioxide, substantial challenges exist for the development of synthetic methods for carbon dioxide incorporation into high value materials. Since carbon dioxide is a relatively stable gas, thermodynamic issues can be an issue for some transformations. In addition, carbon dioxide is often unreactive, which presents challenges for developing rapid catalytic processes for the activation and functionalization of carbon dioxide. In collaboration with the group of Dan Ess (Brigham Young University), we have begun developing catalytic processes to incorporate carbon dioxide into valuable products. Building on our efforts to develop catalysts for olefin hydroarylatio,1 initial projects are directed toward incorporating carbon dioxide into catalytic C–H functionalization processes.
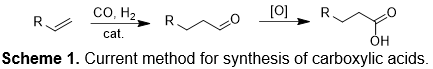
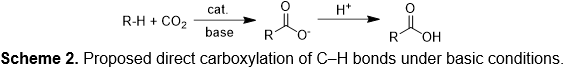
Currently, carboxylic acids are largely prepared by the hydroformylation of alkenes followed by aldehyde oxidation (Scheme 1). In this overall synthetic route for carboxylic acid production, carbon monoxide and strong oxidizing reagents are used. In comparison, the proposed reaction (Scheme 2) for direct carboxylation of C–H bond uses carbon dioxide as the C1 source. But, the at room temperature (298 K) the conversion of benzene and carbon dioxide to benzoic acid is engergonic with ΔGo = 5.85 kcal/mol (at 298 K; ΔHo = -9.91 kcal/mol). Thus, direct carboxylation without base often exhibits both high activation energy and unfavorable free energy. We are exploring strategies to develop catalytic processes with low activation barriers for carbon dioxide functionalization as well as to provide a suitable driving force for endergonic reactions.